Recent interest in reconfigurable materials with responsive electrical, optical, magnetic, mechanical and other properties has led to many exciting studies of molecular switches. The Canary lab focuses on reconfigurable chiral catalysts and has developed a redox-reconfigurable copper complex catalyst derived from L-methionine and urea groups. This catalyst adopts two pseudo-enantiomeric helically chiral states able to invert upon oxidation/reduction of the copper center and produces enantiomeric products. Enantiomeric excess up to 72% (S) and 70% (R) was obtained when the catalyst was applied to a Michael addition reaction. Encouraged by the chiral discrimination of the redox configurable catalyst, the dynamic aspect of the system, the lab is currently studying applications that may benefit from real-time switching in reactions where more than one stereocenter is formed.
Office: (001) 212 998 8422
Email: [email protected]
Mailing Address
Professor James W. Canary
Department of Chemistry
New York University
100 Washington Square East
New York, NY 10013
Lab Location
Centrally located in Greenwich Village, the lab can be easily reached by subway from anywhere in the city.
Reconfigurable Chiral Catalyst
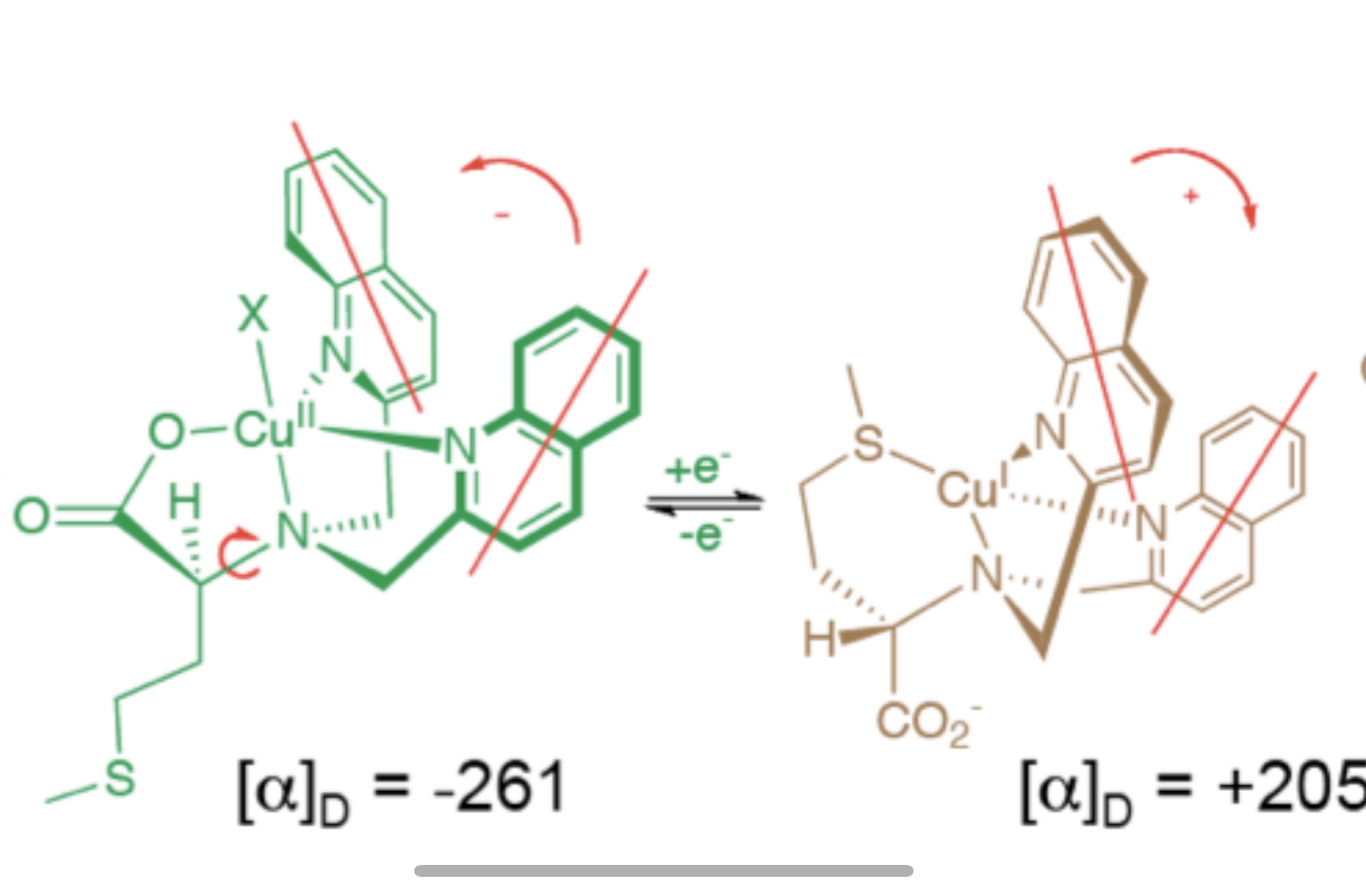
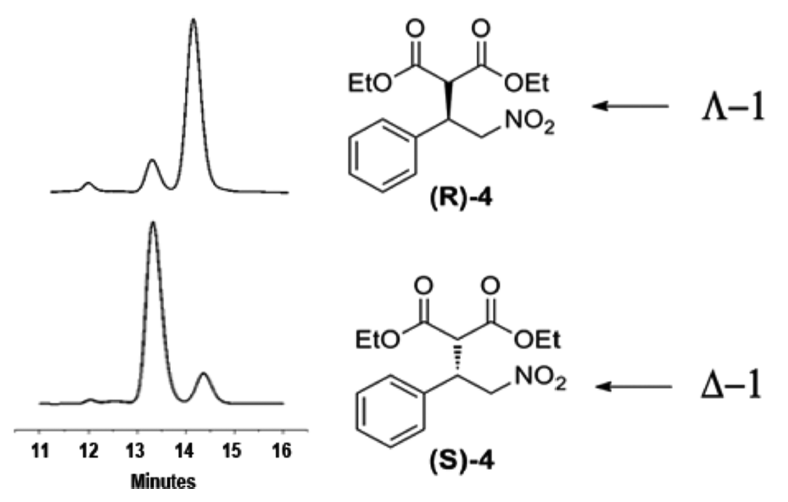
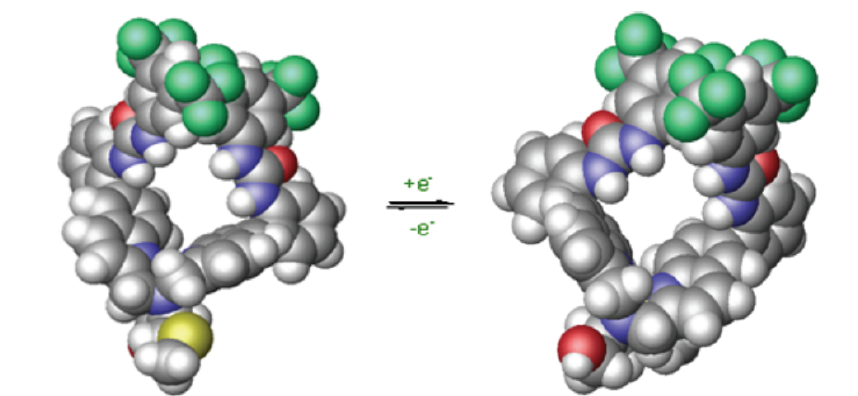
Reading list:
- Mortezaei S, Catarineu NR, Canary JW. Dial-in selection of any of four stereochemical outcomes among two substrates by in situ stereo-reconfiguration of a single ambidextrous catalyst. Tetrahedron Letters 2016; 57: 459-462.
- Mortezaei S, Catarineu NR, Duan X, Hu C, Canary JW. Redox-configurable ambidextrous catalysis: structural and mechanistic insight. Chemical Science 2015; 6: 5904-5912.
- Mortezaei S, Catarineu NR, Canary JW. A redox-reconfigurable, ambidextrous asymmetric catalyst. Journal of the American Chemical Society 2012; 134: 8054-8057.
- Canary JW, Dai Z, Mortezaei S. Spectroscopic Analysis: Chiroptical Sensors Comprehensive Chirality. Comprehensive Chirality 2012; 8: 600-624.
- Liang, J and Canary JW. A Stereodynamic Tripodal Ligand with Three Different Coordinating Arms: Synthesis and Zinc(II), Copper(I) Complexation Study. Chirality 2011; 23: 24-33.
- Zahn S, Canary JW. Electron-induced inversion of helical chirality in copper complexes of N,N-dialkylmethionines. Science 2000; 288: 1404-7.
